
Quality and Safety
Our Quality and Safety Policy
We see our responsibility as being two-fold: firstly, we have a responsibility for the safety and quality of our products, and we also have a responsibility to contribute to making society a place where everyone is safe and can live a healthy life.
To achieve this, we have established a Quality Policy and Action Plan Guidelines, and Food Safety Policy and Action Plan Guidelines based on the quality management laws and required international certification standards. We situate ISO9001 as our core policy underpinning our quality management system so that we can improve the quality of our products as well as our business operations.
Quality Policy and Action Plan Guidelines
Quality Policy
We are committed to a quality management system and to continuously improving it to increase customer satisfaction and to make a positive contribution to the economy and society as a whole.
Action Plan Guidelines
- We are attentive to our customers and strive to provide them with new value and services.
- We are committed to safe and secure manufacturing methods and products for both people and the natural environment.
- We establish quality-related objectives, and implement and review initiatives to achieve them.
- We are committed to complying with all laws and regulations related to quality.
- We facilitate smooth communication with stakeholders and within the Company regarding quality information.
- We promote education and human resource development to cultivate and instill a culture of prioritizing quality.
Food Safety Policy and Action Plan Guidelines
Food Safety Policy
We are committed to providing safe, high-quality products and earning the trust of our customers.
Action Plan Guidelines
- As an integral part of the food chain, we continuously improve our food safety management system.
- We establish food safety-related objectives, and implement and review initiatives to achieve them.
- We are committed to complying with all laws and regulations related to food safety and meeting the requirements of our customers.
- We facilitate smooth communication with stakeholders and within the Company regarding food safety information.
- We deepen our understanding of the importance of food safety, improve our knowledge, and cultivate and instill a culture of food safety.
Quality Assurance System
At Nagase Viita, we manufacture and sell products in a variety of fields, primarily centered around saccharides, and have established a quality assurance system to meet the quality requirements specific to each field.
For example, the food and food additives, such as TREHA™, SANMALT™, PULLULAN and enzymes produced at the Okayama Functional Saccharide Plant, Okayama Plant II, and Fukuchiyama Plant which have obtained FSSC22000 certification under the Global Food Safety Initiative (GFSI) Approval Scheme, in addition to ISO9001 certification. These products are manufactured not only with a focus on quality but also under hygiene management based on HACCP. Food production at other plants has also established a food safety management system in compliance with these standards.
Further, the Okayama Plant II manufactures maltose, an active pharmaceutical ingredient, and trehalose products used as pharmaceutical additives, while the Fujita Pharmaceutical Plant manufactures Lumin™, a low-risk OTC pharmaceutical (as classified in Japan). These facilities comply with GMP (Global Manufacturing Practice) standards for pharmaceutical manufacturers under the Pharmaceuticals and Medical Devices Act. In the case of Lumin™, we have also established a system for quality assurance and safety management in the market as a licensed company in the Marketing and Manufacturing of Pharmaceuticals under the same law. Alongside efficient operation of these systems, we continuously improve our quality management system and provide safe, high-quality products to our customers. Many of our products are also certified Halal and Kosher, and we have additionally obtained certifications such as COSMOS, which relate to the use of naturally derived, environmentally friendly raw materials.
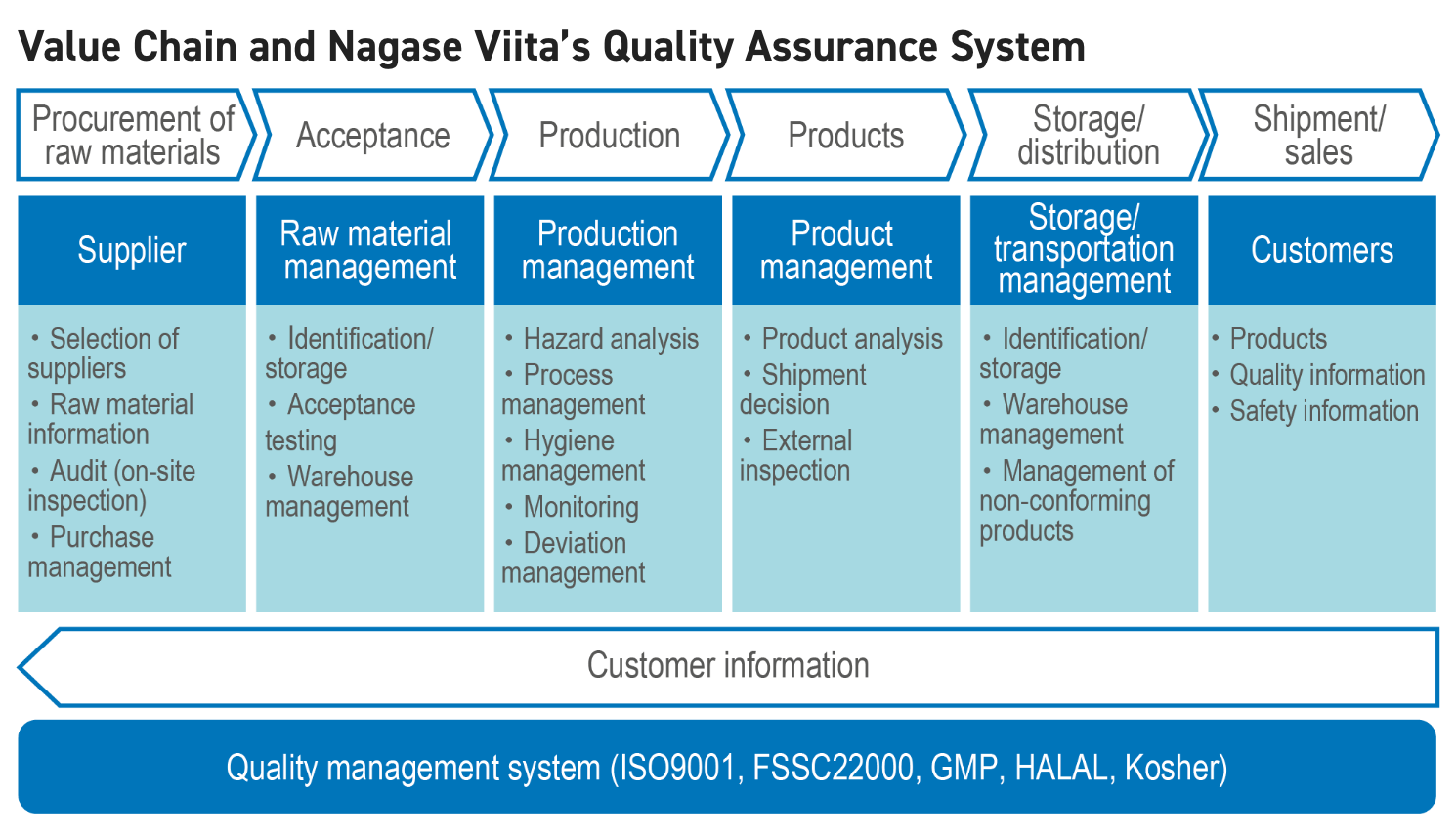
Initiatives for Quality Control
In order to deliver safe and reliable products through the operation of the quality management systems such as ISO9001 and FSSC22000, Nagase Viita implements quality control at each stage of the supply chain, from the reception of raw materials to the shipment of products. We also ensure the quality assurance system, including confirmation by periodic internal audits, and security in product traceability.
Raw Material Management
To manufacture safe and quality products, we purchase the raw material from selected suppliers. We obtain raw material information (GMO, non-GMO raw materials, residual pesticide and allergens, etc.), and check each accepted load to ensure the material has met compliance with our in-house raw materials specifications. In addition, we periodically review the suppliers through audits of their quality management and quality assurance systems.

Production Management
To ensure stable product quality, we manage each production process to ensure compliance with our designated standards. We employ closed-system production lines to prevent contamination and take the needed contamination countermeasures such as installation of sieves, magnetic foreign object removal devices, and metal detectors in the production lines.The hygienic environment of the production facilities is checked by periodic evaluation of the working environment.

Product Release Management
After confirming that each product lot has been properly manufactured in the production process, we conduct physicochemical and hygiene tests in accordance with quality standards, and ship products that pass these quality standards.

Management systems
| Location name | ISO9001 | FSSC22000 | Others |
|---|---|---|---|
| Okayama Plant Ⅰ | 〇 | 〇 (transglucosylation products) | |
| Okayama Plant Ⅱ | 〇 | 〇 (PULLULAN and other items) | Pharmaceutical GMP |
| Okayama Functional Saccharide Plant | 〇 | 〇 | |
| Fujita Pharmaceutical Plant | Pharmaceutical GMP | ||
| Fukuchiyama Plant | 〇 | 〇 | Food additives GMP |