Our Journey
1883
Founded as Hayashibara Shoten (a starch syrup manufacturer) in Okayama, Japan. 
1932
- Hayashibara Shoten was incorporated on July 10.
1935
- Developed the two-step (acid and malt) starch-conversion method for starch syrup production.
1936
Increased daily output of starch syrup to 40 metric tons. 
1943
- The company's name was changed from Hayashibara Shoten to Hayashibara Co., Ltd.
1945
- Entire factory destroyed by an air raid.
1946
- Rebuilt factory and resumed operations.
1947
- Established Japanese Research Institute for Photosensitizing Dyes Co., Ltd.
1950
Increased the daily output of syrup to 150 metric tons, making Hayashibara the largest starch syrup manufacturer in Japan. 
1951
- Japanese Research Institute for Photosensitizing Dyes Co., Ltd. launched pharmaceutical products LUMIN™ A-50 γ tablet and LUMIN™ A-100 γ tablet containing a photosensitizing dye as an active ingredient.
1959
Industrialized the enzymatic production method of glucose from starch. 
1960
- Constructed a new factory in Okayama, Japan to process 180 metric tons of glucose daily.
1962
- Established Hayashibara Shoji, Inc. (Trading Company) for expanding sales of starch syrup and glucose.
1965
- Developed the enzymatic saccharification method for maltose.
1967
- Discovered Isoamylase, a debranching enzyme that acts on the branching parts of starch molecules (amylopectin).
1968
Developed the production method for high purity maltose (not less than 99%). 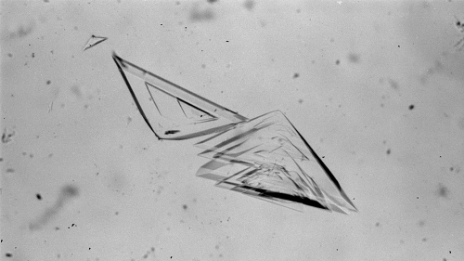
- Developed the production method for maltitol and licensed it to Anic S.p.A. in 1979.
1969
- Developed the production method for glycosylsucrose syrup incorporating the characteristics of sucrose with other properties.
1970
- Established Hayashibara Biochemical Laboratories, Inc.
1971
- Developed the production method for cyclodextrin (licensed it to Ensuiko Sugar Refining Co., Ltd. in 1987 and to Novo Nordisk in 1998).
1972
- Developed the production method for pullulan, a polysaccharide thickener and edible film matrix.
1974
Completed Okayama Plant II in Okayama, Japan. 
- Launched SUNMALT™, food grade maltose powder.
1976
- Began manufacturing PULLULAN in a new production facility at Okayama Plant II.
1977
- Developed the production method for glucosyl stevia (licensed it to Toyo Sugar Refining Co., Ltd. in 1979 and to Sanyo-Kokusaku Pulp Co., Ltd. in 1982).
1978
- Developed the in vivo proliferation method using human cells for the industrial-scale manufacturing of bioactive substances.
1979
- Developed the production method for lactosucrose, an oligosaccharide with unique characteristics.
1981
Completed Fujisaki Institute in Okayama, Japan. 
- Developed the production method for anhydrous crystalline maltitol (licensed it to Towa Chemical Industry Co., Ltd. and Anic S.p.A. in 1982 and to Roquette Frères in 1992).
1983
- The Company marked its 100th anniversary.
1985
- Completed Fujisaki Cell Center in Okayama, Japan.
1987
- Developed the production method for maltotetraose,a starch syrup with pleasant sweetness and reduced off-notes.
- Completed Kibi Pharmaceutical Plant & Institute in Okayama, Japan.
1988
- Was approved to produce bulk interferon-α, and the interferon-α as a therapeutic drug.
- Commercial products were launched by Otsuka Pharmaceutical Co., Ltd. and Mochida Pharmaceutical Co., Ltd.
- Japanese Research Institute for Photosensitizing Dyes Co., Ltd. completed Fujita Plant in Okayama, Japan.
- Their Imperial Highnesses the Crown Prince Akihito and the Crown Princess Michiko (presently the Emperor Emeritus and the Empress Emeritus of Japan) honored Kibi Pharmaceutical Plant with a royal visit.
1989
Developed the production method for ascorbic acid 2-glucoside, a stable vitamin C. 
Developed the production method for glucosyl hesperidin, a citrus-derived polyphenol. 
- Developed the production method for glucosyl rutin and licensed it to Toyo Sugar Refining Co., Ltd.
1993
- Was approved for the extended application of interferon-α drug, OIF, to treat hepatitis B. The commercial product launched by Otsuka Pharmaceutical Co., Ltd.
- Developed the production method for maltosyltrehalose, a carbohydrate with low reducing potential.
1994
Developed the production method for trehalose from starch. 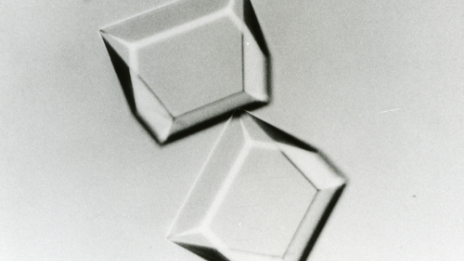
1995
- Discovered Interleukin-18 (IL-18), a new bioactive substance.
- Launched TREHA™, a high purity trehalose.
- Launched AA2G™, a stable vitamin C for cosmetic use.
1997
- Won a patent dispute on biotechnology patent with Roche, a company based in Switzerland.
- Entered into an exclusive supply contract with the U.S. firm Warner-Lambert Company to supply PULLULAN for use in a breath freshening film.
- Japanese Research Institute for Photosensitizing Dyes Co., Ltd. completed Fujita Pharmaceutical Plant in Okayama, Japan.
1999
- Merged Japanese Research Institute for Photosensitizing Dyes Co. and other companies into Hayashibara Biochemical Labs., Inc.