-Bacstain- Series

-Bacstain- Series
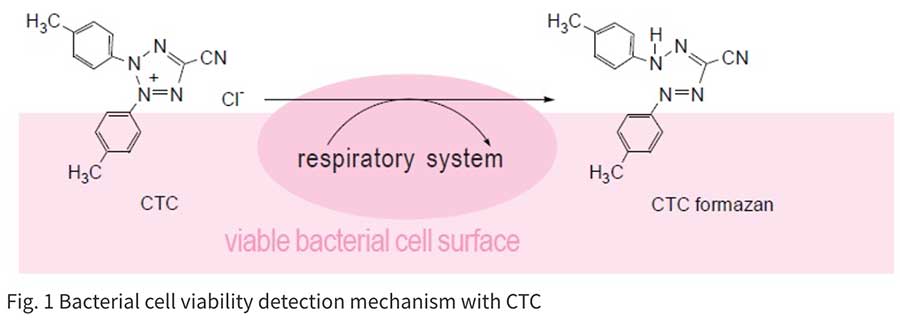
細菌や真菌の生存率は寒天培地を用いたコロニー形成能によって評価されるのが一般的ですが、この手法は長い時間を必要とします(24~72時間)。また環境中に存在する微生物のほとんどがいまだ最適な培養条件が見いだせてないとされています。迅速検出法も発展を続けており、PCR法やLAMP法などの遺伝試験も汎用されています。しかしながら、これらの遺伝子検査では死細胞も検出されてしまうため、生存率を求めることができません。
-Bactain-シリーズのように蛍光染色も迅速検出法のひとつとして、生存率測定に応用されています。
| 製品コード | 製品名 | 容量 | SDS / Protocol |
|---|---|---|---|
| BS01 | -Bacstain- CTC Raoud Staining Kit (for Flow cytomery) | 100 assays | SDSProtocol |
| BS02 | -Bacstain- CTC Raoud Staining Kit (for Microscopy) | 100 assays | SDSProtocol |
| BS03 | -Bacstain- CFDA solution | 100 assays | SDSProtocol |
| BS04 | -Bacstain- DAPI solution | 100 assays | SDSProtocol |
| BS05 | -Bacstain- AO solution | 100 assays | SDSProtocol |
| BS07 | -Bacstain- PI solution | 100 assays | SDSProtocol |
製品情報
微生物染色試薬(-Bacstain- シリーズ)
Technical info
Microscopy detection
1. Centrifuge bacteria culture and remove the supernatant, and then resuspend the bacteria pellet with PBS(-).
2. Add CTC + Enhancing reagent-B. Incubate at 37°C for 1 hour.
3. Prepare a slide and detect fluorescence by B-excitation filter set.
Flow cytometry detection
1. Centrifuge bacteria culture and remove the supernatant, and then resuspend the bacteria pellet with PBS(-).
2. Add CTC + Enhancing reagent-A. Incubate at 37°C for 1 hour.
3. Analyze the cells with a flow cytometry: 488 nm excitation, 630 nm emission.
FACS Data
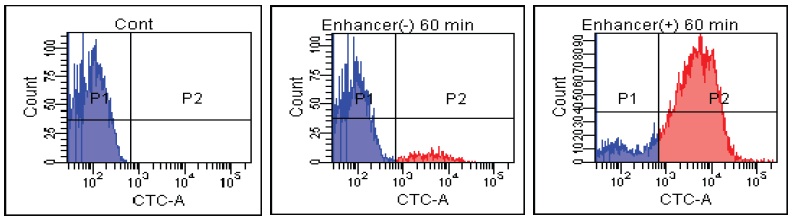
E. coli
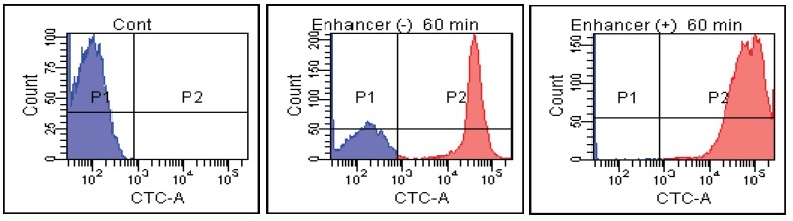
B. Subtilis
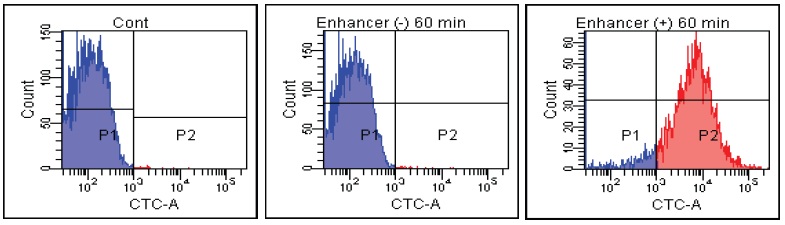
Candida albicans
E. faecalis
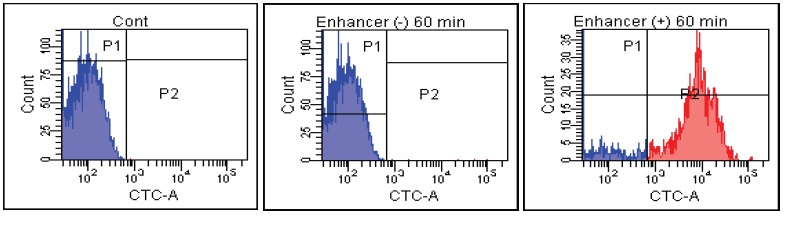
Fig. 2 CTC staining of various microorganisms with and without Enhancing reagent.
Experimental Example
Observation by Microscopy
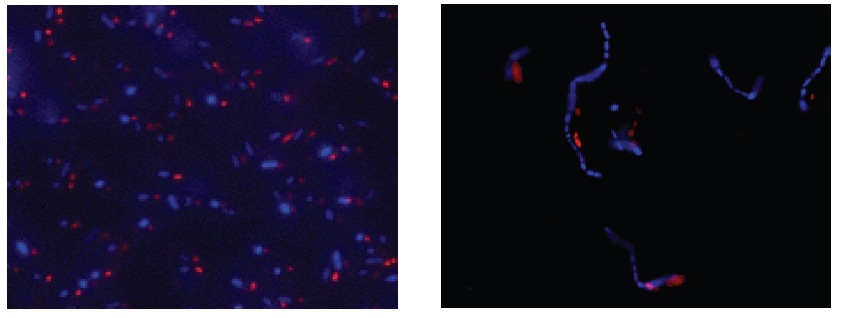
Fig. 3 E. coli staining (left) and L. casei staining (right) with CTC and DAPI. Bacterial cells were stained with CTC Rapid Staining Kit first, and then 1 μl of DAPI solution was added. The cells were incubated at room temperature for 5 min. Formaldehyde fixation with 1-4% formaldehyde can be performed before DAPI staining.
参考文献
2) E. Severin, J. Stellmach and H. -M. Nachtigal, "Fluorimetric Assay of Redox Activity in Cells", Anal. Chim. Acta, 1985, 170, 341.
3) G. G. Rodriguez, D. Phipps, K. Ishiguro and H. F. Ridgway, "Use of a Fluorescent Redox Probe for Direct Visualization of Actively Respiring Bacteria", Appl. Environ. Microbiol., 1992, 58(6), 1801.
4) G. Schaule, H. C. Flemming and H. F. Ridgway, "Use of 5-Cyano-2,3-ditolyl Tetrazolium Chloride for Quantifying Planktonic and Sessile Respiring Bacteria in Drinking Water", Appl. Environ. Microbiol., 1993, 59(11), 3850.
5) R. A. Bovill, J. A. Shalloross and B. M. Markey, "Comparison of the Fluorescent Redox Dye 5-Cyano-2,3-ditolyltetrazolium Chloride with p-Iodonitrotetrazolium Violet to Detect Metabolic Activity in Heat-stressed Listeria monocytogenes Cells", J. Appl. Bacteriol., 1994, 77(4), 353.
6) M. T. E. Suller and D. Lloyd, "Flow Cytometric Assesment of the Postantibiotic Effect of Methicillin on Staphylococcus aureus", Antimicrob. Agents Chemother., 1998, 42(5), 1195.
7) M. Kawai, N. Yamaguchi and M. Nasu "Rapid Enumeration of Physiologically Active Bacteria in Purified Water Used in the Pharmaceutical Manufacuturing Process", J. Appl. Microbiol., 1999, 86, 496.
8) N. Yamaguchi, M. Sasada, M. Yamanaka and M. Nasu, "Rapid Detection of Respiring Escherichia coli O157:H7 in Apple Juice, Milk and Ground Beef by Flow Cytometry", Cytometry , 2003, 54A, 27.
9) A. Kitaguchi, N. Yamagauchi and M. Nasu, "Enumeration of Respiring Pseudomonas spp. in Milk within 6 Hours by Fluorescence In Situ Hybridization Following Formazan Reduction", Appl. Environ. Microbiol., 2005, 71(5), 2748.
10)A. Hiraishi and N. Yoshida, "An Improved Redox Dye-Staining Method Using 5-Cyano-2,3-Ditoryl Tetrazolium Chloride for Detection of Metabolically Active Bacteria in Activated Sludge", Microbes Environ., 2004, 19(1), 61.