Epoxy Crosslinker: The Application as a “Crosslinking Agent” for Epoxy Resins
1. What Is a Crosslinking Reaction?
A crosslinking reaction is a process that chemically binds polymers to each other, much like building a bridge. By crosslinking polymers, the chemical structure becomes more robust, improving physical and chemical properties.

Crosslinking reactions are an effective method for improving various properties, including water resistance and chemical resistance in polymer materials, such as resins and paints.
A crosslinking agent is necessary for a crosslinking reaction. However, there is a vast array of crosslinking agents, each with different characteristics and properties depending on their chemical structure. It is necessary to choose the appropriate crosslinking agent that corresponds to the properties of the polymer to be crosslinked.
The reactivity of the crosslinking agent is dependent on functional groups. These groups are the starting points of crosslinking reactions and include such types as hydroxyl groups (-OH), carboxyl groups (-COOH) and amino groups (-NH2). There are also crosslinking agents with multiple functional groups, and the chemical structure formed changes according to the number and type of functional groups, which in turn alters the resulting properties.
Therefore, an appropriate cross-linking agent for the purpose and application is required for the cross-linking reaction.
2. What Is an Epoxy Crosslinker?
An epoxy crosslinker is an epoxy compound that contains two or more epoxy groups, possessing excellent characteristics as a crosslinking agent. An epoxy group consists of a three-membered ring structure made up of two carbon atoms and one oxygen atom, and it is known for its very high reactivity.
Let's examine some familiar examples of epoxy crosslinkers, crosslinking reactions, and crosslinking density.
(1) Common Examples Where Epoxy Crosslinkers Are Used
Epoxy crosslinkers are used in various applications. For example, they are used to improve the properties of various polymer materials, such as acrylic resins used in diapers, adhesives for protective films, and paints.
In addition, they are also used in treatments for fabrics to impart wrinkle resistance to handkerchiefs and shirts and in resin modifiers to impart hydrophilic or hydrophobic properties to resins.

(2) How Does a Crosslinking Reaction Occur?
Crosslinking reactions with epoxy crosslinkers occur when the epoxy group and the functional group (with active hydrogen) of the target polymer bind together due to heat or light (ultraviolet rays).
Also, the reactivity varies with the number of epoxy groups and the chemical structure of the epoxy compound. The reaction rate of the crosslinking reaction and the properties of the reaction products vary depending on the structure of the epoxy compound used, the solvent, and the reaction conditions.
(3) What Is Crosslinking Density?
Crosslinking density refers to the proportion of starting points (crosslinking points) for crosslinking within the polymer that has undergone the crosslinking reaction. A higher crosslinking density results in stronger binding and improved durability and strength. Conversely, lower crosslinking density improves flexibility.
The crosslinking density can be controlled by the number of functional groups and by selecting the length of the crosslinker molecule (chain length).
3. Benefits Brought About by Crosslinking Reaction
Crosslinking reactions can improve various properties, such as water resistance and solvent resistance, by increasing the molecular weight of polymers and changing their chemical structure. It is also possible to impart functions such as flexibility and water solubility.
The benefits brought by crosslinking reactions are abundant, although they vary depending on the crosslinking agent used and the properties of the compound to be crosslinked.
As an example, let's take a look at the effect of using a special epoxy compound Denacol(https://denacol.com/en/feature/), developed by Nagase ChemteX, as a crosslinking agent for paints.
(1) Improving Adhesion to the Substrate
When Denacol was added to paint based on waterborne or solvent-borne acrylic resin and applied to substrates, such as tin or 6-nylon, an obvious improvement in the adhesion of the paint was observed.
This is because Denacol, as an epoxy crosslinker, caused a crosslinking reaction with the resin of the paint, improving its adhesion to the substrate.
(2) Improvement in Water Resistance of the Coating Film
In the above-mentioned water resistance test, the appearance changed significantly in the absence of Denacol, but there was hardly any change when Denacol was added. This suggests that the water resistance of the coating film was improved by the crosslinking reaction.
(3) Improvement of Solvent Resistance of the Coating Film
Similarly, it has been found that the solvent resistance improves when Denacol is added.
4. Examples of Crosslinking Reactions
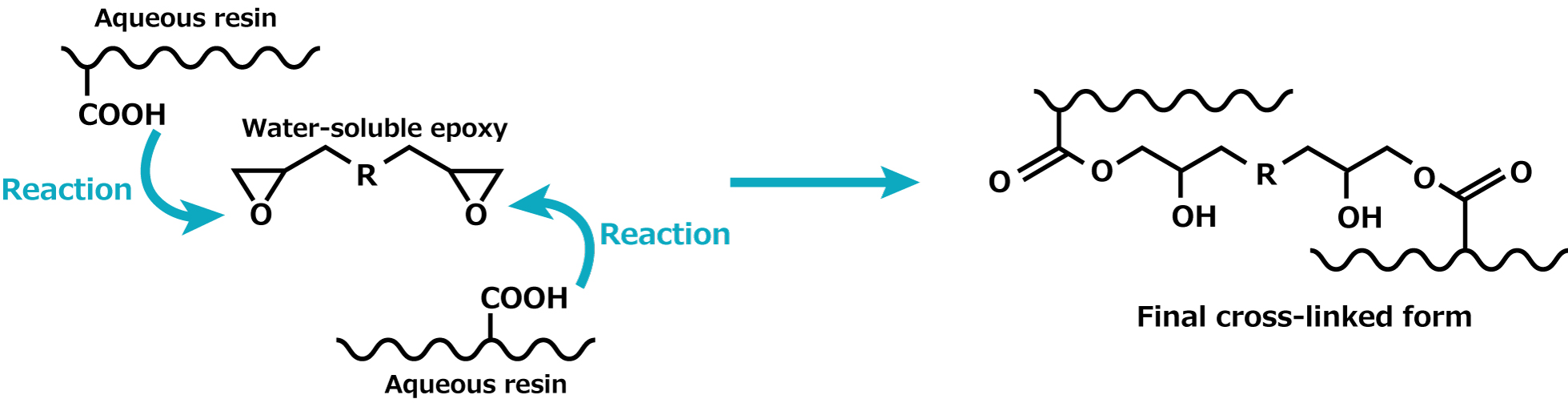
Let's introduce some specific examples of crosslinking reactions. The diagram below represents the crosslinking reaction between a water-soluble epoxy compound and a water-based resin. The epoxy groups at both ends of the water-soluble epoxy compound and the carboxyl groups (-COOH), which are the functional groups of the water-based resin, undergo an addition reaction to form a crosslinked structure.
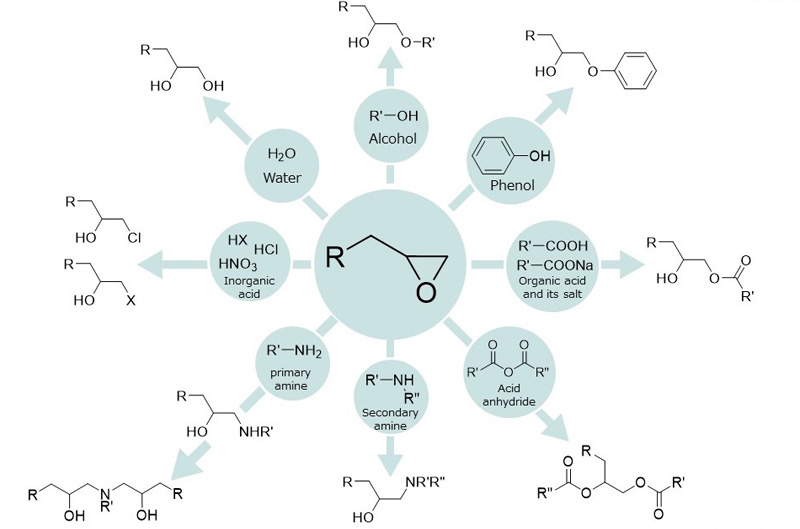
In addition, similar crosslinking reactions occur with compounds such as alcohols, phenols, acid anhydrides, primary and secondary amines, inorganic acids, and water.
5. How To Select Epoxy Crosslinkers
The selection criteria for epoxy crosslinkers are based on the following five points. Please use these as references when selecting epoxy crosslinkers suitable for the properties of the compounds you want to crosslink and the characteristics you want to improve through crosslinking, according to the application and purpose.
(1) Water-soluble or hydrophobic.
(2) The functionality you want to impart (e.g., flexibility, film hardness, water resistance, solvent resistance, or adhesion).
(3) Reaction methods (e.g., reaction temperature during thermal curing or wavelength during photopolymerization).
(4) Type of catalyst.
(5) Self-crosslinking or not.
6. Denacol: A Spectrum of Solutions for Crosslinking Needs
Denacol, developed by Nagase ChemteX, offers a rich lineup to accommodate various applications. Broadly classified based on the number of epoxy groups, there are three types: multifunctional, bifunctional, and monofunctional.
Multi-functional type
The multifunctional type is composed of epoxy compounds with a backbone of substances, such as sorbitol, glycerin, or pentaerythritol. It is suitable for use as a crosslinker for resins, improving the adhesion of coatings, and modifying fibers and paper.
Most are water-soluble, making them suitable for water-related operations, such as the production of fibers and paper. There are environmentally friendly crosslinkers, such as VOC-free. The product names include the “EX-300, 400, 500, 600 series.”
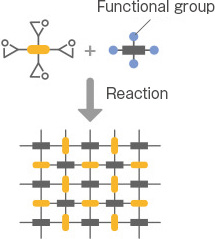
Di-functional type
The multifunctional type is composed of epoxy compounds with a backbone of substances, such as sorbitol, glycerin, or pentaerythritol. It is suitable for use as a crosslinker for resins, improving the adhesion of coatings, and modifying fibers and paper.
Most are water-soluble, making them suitable for water-related operations, such as the production of fibers and paper. There are environmentally friendly crosslinkers, such as VOC-free. The product names include the “EX-300, 400, 500, 600 series.”
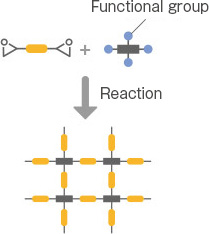
Mono-functional type
The monofunctional type is composed of monofunctional epoxy compounds with a backbone of substances, such as 2-ethylhexanol or phenol. It is suitable for use as a reactive diluent or resin stabilizer.
It comes in both hydrophilic and hydrophobic types, and you can choose based on the purpose and situation. The product names include the “EX-100 series.”
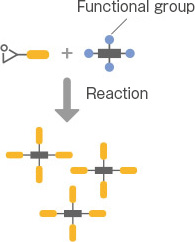
5. How To Select Epoxy Crosslinkers
Below, we will introduce the characteristics and physical properties of representative products. Please use them according to the application and purpose.
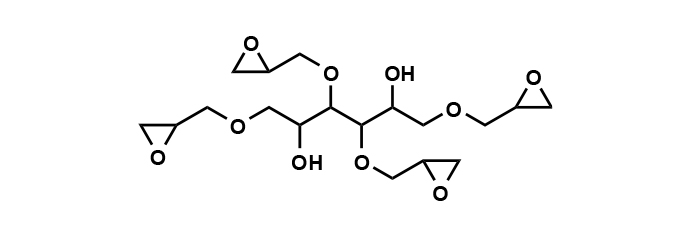
EX-614B: The Multifunctional Epoxy Dynamo
EX-614B is a multifunctional aliphatic epoxy compound, known as sorbitol polyglycidyl ether. Its prowess lies in forming a robust three-dimensional crosslinked structure that can be the catalyst for enhanced dimensional stability, heat resistance, and chemical resistance. With its water-soluble and highly reactive nature, EX-614B excels as a crosslinking agent in aqueous systems and as a surface treatment agent for fibers. Key physical properties of EX-614B include an epoxy equivalent of 173 (g/eq.), a viscosity of 5,000 (mPa·s), total chlorine content of 10.1%, and water solubility of 94%.
SWIPE
| Epoxy equivalent (g/eq.) |
Viscosity (mPa・s) |
Total chlorine content(%) |
Color value (APHA) |
Water solubility(%) | Packaging |
|---|---|---|---|---|---|
| 173 | 5,000 | 10.1 | 3 | 94 | 20kg、200kg |
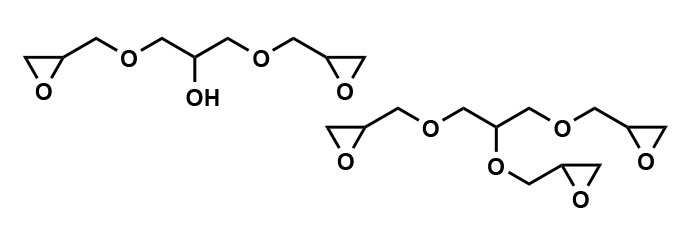
EX-313: The Agile Performer in Multifunctional Epoxy Compounds
EX-313, also known as glycerol polyglycidyl ether, is another multifunctional aliphatic epoxy compound similar to EX-614B but with a unique twist; it features a low viscosity. As with EX-614B, EX-313 is water-soluble and highly reactive, making it a prime choice for use as a crosslinking agent in water-based paints and adhesives, as well as a surface treatment agent for fibers. Notable physical properties of EX-313 include an epoxy equivalent of 141 (g/eq.), a viscosity of 150 (mPa·s), total chlorine content of 9%, and water solubility of 99%.
SWIPE
| Epoxy equivalent (g/eq.) |
Viscosity (mPa・s) |
Total chlorine content(%) |
Color value (APHA) |
Water solubility(%) | Packaging |
|---|---|---|---|---|---|
| 141 | 150 | 9 | 10 | 99 | 20kg、200kg |
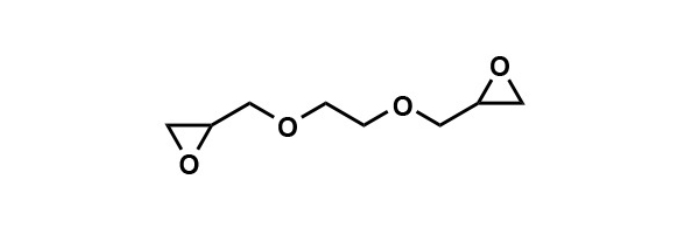
EX-810: The Flexibility Maestro in Epoxy Compounds
EX-810, known as ethylene glycol diglycidyl ether, is a difunctional aliphatic epoxy compound. What sets EX-810 apart is its ability to produce more flexible cured products due to the reduction in crosslinking density, which results in improved brittleness. Its water solubility, low viscosity, low chlorine content, and flexibility make it an excellent choice as a crosslinking agent for water-based resins, hydrophilicity imparting agent, and flexibility imparting agent. Key physical properties of EX-810 include an epoxy equivalent of 113 (g/eq.), a viscosity of 20 (mPa·s), total chlorine content of 0.6%, and water solubility of 100%.
SWIPE
| Epoxy equivalent (g/eq.) |
Viscosity (mPa・s) |
Total chlorine content(%) |
Color value (APHA) |
Water solubility(%) | Packaging |
|---|---|---|---|---|---|
| 113 | 20 | 0.6 | 20 | 100 | 20kg、200kg |
7. Model Composition
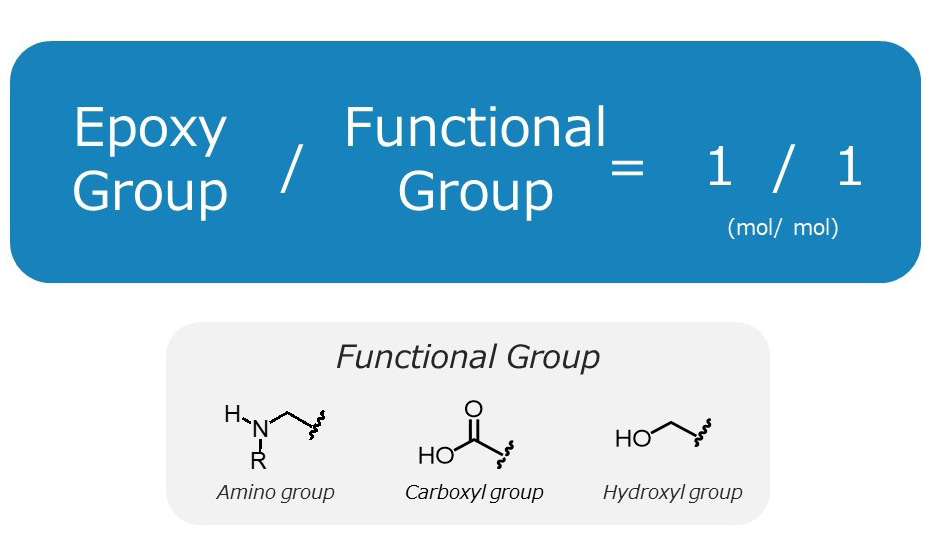
The recommended additional amount of Denacol is such that the mole ratio of the epoxy groups to functional groups is 1:1. Please add it in amounts that make the functional groups in the base resin, such as amino groups, carboxyl groups, and hydroxyl groups, equivalent to the epoxy groups.
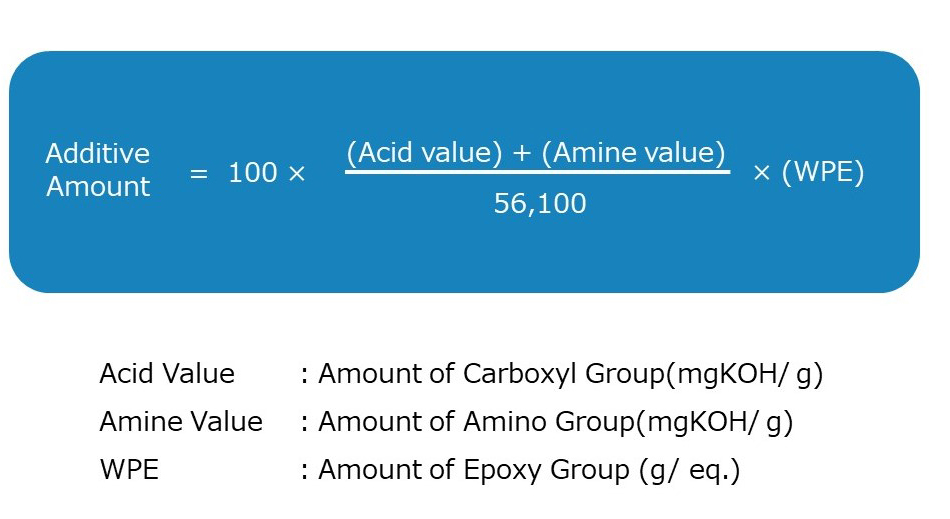
Additionally, the calculation method for the additional amount is shown in the diagram below. It can be determined using the sum of the equivalent of carboxyl groups (measured in mgKOH/g), known as the “acid value,” and the equivalent of amino groups (also measured in mgKOH/g), known as the “amine value,” along with the epoxy equivalent (g/eq.), referred to as the “WPE.”
Please refer to the following as a model composition.