ACE Kit-WST

ACE Kit-WST
The kit is used for the determination of ACE inhibition activity.
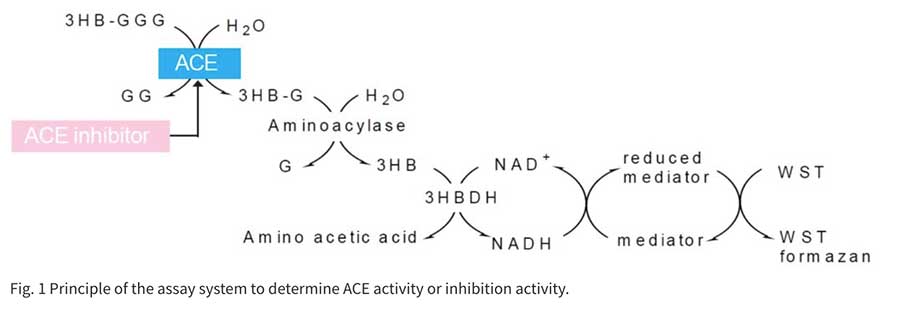
ACE works in the Renin-Angiotensin system, which is one of the mechanisms of blood pressure control, to convert Angiotensin I to the vasopressor Angiotensin II. This enzyme also contributes to elevated blood pressure due to its role in breaking down the antihypertensive peptide Bradykinin. In recent years, food and supplements containing ingredients that block ACE have received attention for their use in preventing high blood pressure. The conventional method of measuring ACE inhibition employs the synthetic substrate Hippuryl-His-Leu. Hippuric acid from the synthetic substrate is extracted with ethyl acetate, condensed, redissolved, and then read at an absorbance of 228 nm. This method is cumbersome and measurement is subjected to error due to residual ethyl acetate. ACE inhibition Assay Kit enzymatically detects 3-Hydroxybutyric acid (3HB), which is made from 3-Hydryoxybutyryl-Gly-Gly-Gly (3HB-GGG). Using a 96-well format, it is possible to test multiple samples at one time. In addition, there is no need to use harmful organic solvents, resulting in a safe, simple, and highly reproducible assay.」
Product information
ACE inhibitory activity measurement kit
Technical info
plate reader with 450 nm filter; 96-well culture plate, 2-20 μl, 20-200 μl, 100-1000 μl and multi-channel pipettes; 37ºC incubator, Disposable syringe (1 ml)
Required Equipment and Materials
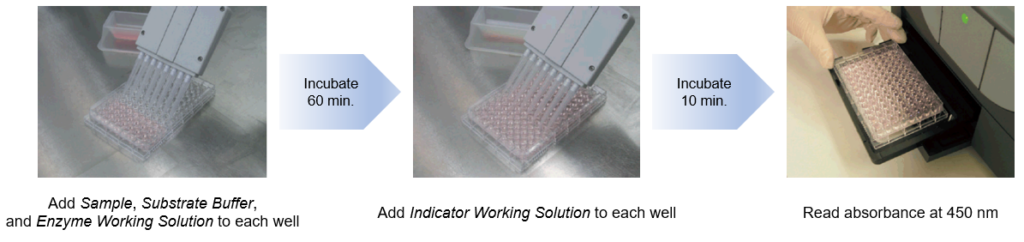
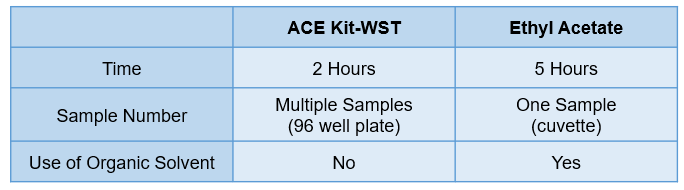
Comparison to the Conventional Method by Ethyl Acetate
Example Assay Data
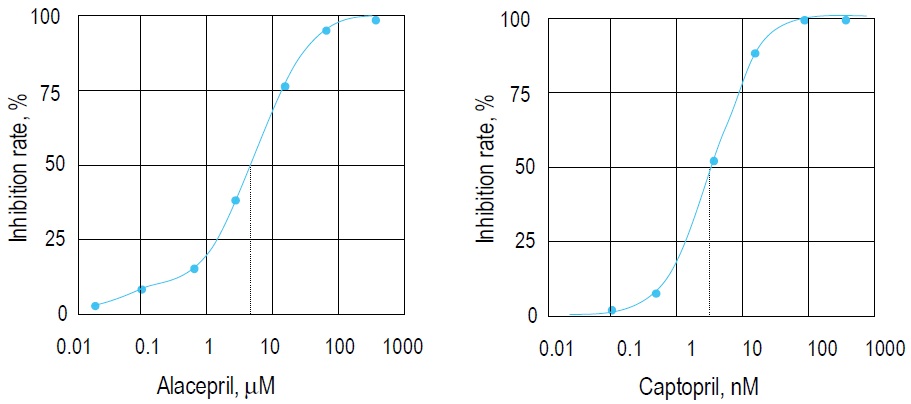
Fig. 2 Inhibition curves prepared by Alacepril and Captopril.
IC50 of Alacepril and Captopril are 3.62 μM and 2.14 nM, respectively. Both compounds are ACE inhibitors.
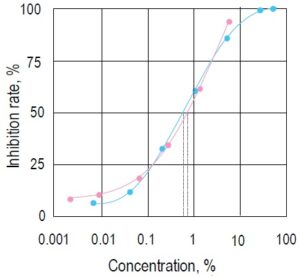
Fig. 3 Inhibition curves prepared by two beverages containing a valyltyrosine (pink●) or lacto tripeptide (blue●) .
IC50 of these beverages are 0.56% and 0.69%, respectively. It is known that these substances have antihypertensive effects. *Concentration of the beverage in the sample solution.
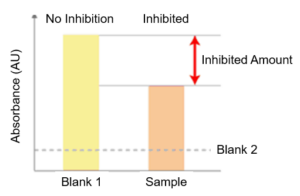
Checking the Presence of ACE Inhibition
ACE inhibitions is present in the sample if absorbance of the sample (Total sample AU – Blank 2 AU) is lower than Blank 1 (Total Blank 1 AU – Blank 2 AU).
For 50 tests unit size, 14 samples can be tested in triplicate
Protocol: Presence of ACE Inhibition
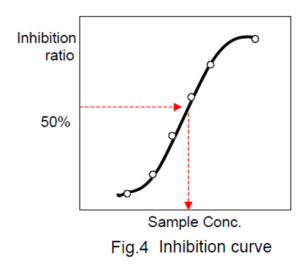
Determination of IC50 (50% inhibitory concentration)
Prepare an inhibit ion curve using the sample concentration for X axis and the ACE inhibitory activity for Y axis. A typical inhibition curve is shown in Fig. 4.
Determine the concentration of the sample solution that gives 50% ACE inhibitory activity as indicated in Fig. 4.
For 50 tests unit size, 2 samples can be tested in triplicate
Protocol: Determination of IC50
The Potential of ACE Kit-WST in Discovery of Angiotensin-Converting Enzyme (ACE) Inhibitors to Suppress Inflammation after Viral Infection (COVID-19)
ACE Kit-WST is expected to be an advantageous tool to support the discovery of ACE inhibitors effective at suppressing inflammation after SARS-CoV-2 infection.
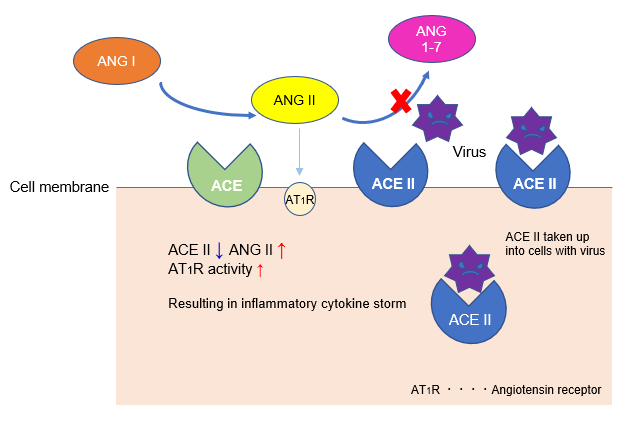
Angiotensin-converting enzyme (ACE) produces angiotensin II (Ang II), a vasoconstrictor, from the C-terminus of angiotensin I (Ang I), which has almost no biological activity. ACE and Ang II activity is countered by ACE2, which functions to cleave inflammatory Ang II into non-inflammatory peptide Ang 1-7.
During SARS-CoV-2 infection, angiotensin-converting enzyme 2 (ACE2) from the cell membrane is taken up into cells along with the virus, decreasing ACE2 activity on the cell membrane. As a result, angiotensin II (Ang II), which had been counteracted by ACE2, becomes uninhibited, beginning a cascade towards an inflammatory cytokine storm that has been suggested to promote lung damage.
Thus, Ang-II mediated cytokine storm is considered to be an important mechanism of severe COVID-19. Therefore, ACE inhibitors, which suppress the Ang II system, are currently expected to reduce the risk of severe Covid-19 symptoms brought on by inflammation, such as acute respiratory distress.
References
1. Hoffmann M, Kleine-Weber H, Schroeder S, et al., “Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor”, Cell, 2020, 181, 271-280 e278.
2. Imai Y, Kuba K, Rao S, et al., “Angiotensin-converting enzyme 2 protects from severe acute lung failure” Nature, 2005, 436, 112-116.
3. Zou Z, Yan Y, Shu Y, et al., “Angiotensin-converting enzyme 2 protects from lethal avian influenza a h5n1 infections”, Nat Commun., 2014, 5, 3594.
4. Gu H, Xie Z, Li T, et al., “Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus”, Sci Rep., 2016, 6, 19840.
References
2. L. H. Lam, et al., Assay of angiotensin I-converting enzyme-inhibiting activity based on the detection of 3-hydroxybutyrate with water-soluble tetrazolium salt. Anal Sci. 2008;24:1057-1060.
3. L. H. Lam, et al., Flow injection analysis of angiotensin I-converting enzyme inhibitory activity with enzymatic reactors. Talanta. 2009;79:1130-1134.
4. Hiromichi Nakamura, et al., Antihypertensive Effects of Continuous Oral Administration of Nattokinase and Its Fragments in Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2011; 34(11) 1696 E701.
5. C. C. Lau, N. Abdullah and A. S. Shuoib, Novel angiotensin I-converting enzyme inhibitory peptides derived from an edible mushroom,Pleurotus cystidiosus O.K. Miller identified by LC-MS/MS, BMC Complement. Altern. Med., 2013, 13, 313.
6. K. Yamaki, Screening Research Methods for α-glucosidase Inhibitors and Angiotensin-converting Enzyme Inhibitors in Fermented Soybean Products and Fermented Milk Products ,JARQ, 2014, 48, 41.
7. C.Lau, N. Abdullah, A. Shuib, and N.Aminudin, Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbachi identified by LC-MS/MS, Food Chemistry, 2014, 148, 396-401.
8. R. Nakabayashi, Z. Yang, T. Nishizawa, T. Mori and K. Saito, Top-down Targeted Metabolomics Reveals a Sulfur-Containing Metabolite with Inhibitory Activity against Angiotensin-Converting Enzyme in Asparagus officinalis, J. Nat. Prod., 2015, 78(5), 1179.
9. M. Alauddin, H. Shirakawa, K. Hiwatashi, A. Shimakage, S. Takahashi, M. Shinbo and M. Komaia, Processed soymilk effectively ameliorates blood pressure elevation in spontaneously hypertensive rats,J. Funct. Foods., 2015, 14, 126.
10. L. Basirico, E. Catalani, P. Morera, S. Cattaneo, M. Stuknyte, U. Bernabucci, I.De Noni, and A. Nardone, Release of angiotensin converting enzyme-inhibitor peptides during in vitro gastrointestinal digestion of Parmigiano Reggiano PDO cheese and their absorption through an in vitro model of intestinal epithelium, Journal of Dairy Science, 2015, 98(11), 7595-7601.
11. T. Hagi, M. Kobayashi, and M. Nomura, Metabolome analysis of milk fermented by gamma-aminobutyric acid-producing Lactococcus lactis, Journal of Dairy Science, 2016, 99(2), 994-1001.