3–HYDROXYBUTYRATE DEHYDROGENASE [3–HBDHⅡ]
from Alcaligenes faecalis
(D–3–Hydroxybutyrate: NAD+ oxidoreductase, EC 1.1.1.30)
D–3–Hydroxybutyrate + NAD+ → Acetoacetate + NADH + H+
Preparation and Specification
- Appearance
- : White amorphous powder, lyophilized
- Specific activity
- : More than 1,500 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 60±5 kDa (TSK G–3000SW)
30±5 kDa (SDS–PAGE)
- Isoelectric point
- : pH 5.0±0.2
- Michaelis constants
- : D–3–Hydroxybutyrate 1.6 × 10-3M
- Optimum pH
- : 8.5Figure 1
- pH stability
- : 5.5–11.0 (37℃, 60 min) Figure 2
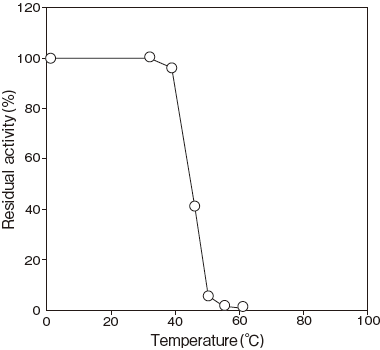
- Optimum temperature
- : 45℃ (Tris–HCl buffer) Figure 3
- Thermal stability
- : Stable at 37℃ and below (pH 8.5, 10 min) Figure 4
- Effect of metal ions
- : See Table 2
- Effect of detergents
- : See Table 3
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of ketone bodies when coupled with acetoacetate decarboxylase (AADC) , thio–NAD and NADH.

Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| 3–Hydroxybutyric acid | 100 |
| 2–Hydroxybutyric acid | 0 |
| D,L–Lactic acid | 0 |
| D,L–Malic acid | 0 |
| Gluconic acid | 0 |
| Glycolic acid | 0 |
Table 2. Effect of metal ions on 3–HBDH Ⅱ activity
| Substrate | Relative activity (%) |
|---|---|
| None | 100 |
| LiCl | 104 |
| NaCl | 101 |
| NH4Cl | 101 |
| KCl | 98 |
| CsCl | 100 |
| CuCl2 | 13 |
| BaCl2 | 107 |
| ZnCl2 | 88 |
| PbCl2 | 60 |
| NiCl2 | 49 |
| CoCl2 | 44 |
| MnCl2 | 40 |
| CaCl2 | 91 |
| MgCl2 | 94 |
| FeSO4 | 91 |
| FeCl3 | 103 |
| EDTA | 85 |
| NaN3 | 102 |
Table 3. Effect of detergents on 3–HBDH Ⅱ activity
| Detergent (0.1%) | Relative activity (%) | |
|---|---|---|
| None | 100 | |
| Pluronic | L–71 | 57.3 |
| P–103 | 94.7 | |
| F–68 | 68.7 | |
| Adekatol | SO–120 | 110 |
| LO–7 | 109 | |
| NP–690 | 112 | |
| PC–8 | 93.9 | |
| NP–720 | 54.2 | |
| Nikkol | SL–10 | 62.9 |
| TL–10 | 74 | |
| MGO | 55.7 | |
| TMGO5 | 54.2 | |
| MYO–6 | 75.6 | |
| MYL–10 | 32.8 | |
| BL–20TX | 101 | |
| NP–18TX | 99.2 | |
| OP–10 | 104 | |
| HCD–100 | 91.6 | |
| TX–100 | 100 | |
| Tween 80 | 65.6 | |
Fig.1 pH Optimum
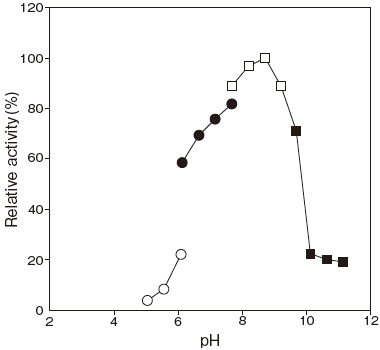
〇: Acetate buffer
●: Phosphate buffer
□: Tris-HCI buffer
■: Glycine-NaOH buffer
●: Phosphate buffer
□: Tris-HCI buffer
■: Glycine-NaOH buffer
Fig.2 pH Stability
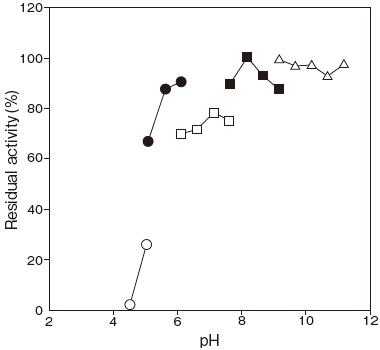
37℃, 60min
〇: Citrate buffer
●: Acetate buffer
□: Phosphate buffer
■: Tris-HCI buffer
△: Glycine-NaOH buffer
〇: Citrate buffer
●: Acetate buffer
□: Phosphate buffer
■: Tris-HCI buffer
△: Glycine-NaOH buffer
Fig.3 Optimum Temperature

pH 8.5
50 mM Tris-HCI buffer
50 mM Tris-HCI buffer
Fig.4 Thermal Stability
pH 8.5, 10min.
50 mM Tris-HCI buffer
50 mM Tris-HCI buffer
Assay
Principle
The assay is based on the increase in absorbance at 340 nm as the formation of NADH proceeds in the following reaction:
| 3–HBDH Ⅱ | ||
| 3–Hydroxybutyrate+NAD+ | → | Acetoacetate+NADH+H+ |
NAD:Nicotineamido adenine dinucleotide
Unit definition
One unit is defined as the amount of enzyme which converts 1 μ mole of 3–Hydroxybutylate to acetoacetate per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
Dissolve 126 mg of D– (–) –3–hydroxybutylic acid with 12.5 ml of 0.2 M Tris–HCl buffer pH 8.5 and add 25 ml of distilled water and 12.5 ml of 10 mM NAD solution. - Enzyme dilution buffer
20 mM Tris–HCl buffer pH 8.5 containing 0.1% (W/V) BSA. - Reagents
NAD: NACALAI TESQUE, INC. #24334–84
D–(–)–3–Hydroxybutylic acid (Na salt) :Sigma Chemical Co. #29836-0BSA: Millipore Fraction V pH5.2 #81–053
Enzyme solution
- Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it
- with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 3.0 ml of reaction mixture into a small test tube and preincubate at 37℃.
- After 5 min, add exactly 40 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 40 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase per minute in absorbance at 340 nm. The rate must be measured within the linear portion of the absorbance curve.
△A/min = (As/min-Ab/min) ≦ 0.070 Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
- Activity (U/mg of powder) = {(△A/min)/6.22} × 3.04/0.04 × 1/x
6.22: millimolar extinction coefficient of NADH at 340 nm (cm2 /μmole)3.04 : final volume (ml) 0.04 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
Storage at -20℃ in the presence of a desiccant is recommended.
3–HBDH Ⅱ活性測定法 (Japanese)
試薬液
- 反応試薬混合液
3–ヒドロキシ酪酸126mg を0.2M トリス–HCl 緩衝液pH8.5 12.5ml で溶解した後、精製水25ml と10mM NAD 溶液12.5ml を混合する。 - 酵素溶解希釈用液
0.1% (W/V) BSA を含む20mM トリス–HCl 緩衝液pH8.5 - 試薬
NAD (ニコチンアミドアデニンジヌクレオチド):ナカライテスク製 #24334–843– ヒドロキシ酪酸[D– (–) –3– ヒドロキシ酪酸・ナトリウム塩]:シグマ製 #29836-0
BSA: Millipore 製 Fraction V pH5.2 #81–053
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液3.0ml を正確に分注して37℃で予備加温する。
- 5 分経過後、酵素試料液40 μl を加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液40 μl を加える。 - 反応開始後、340nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。
求められた吸光度変化を試料液はAs/min、盲検液はAb/min とする。
ΔA/min = (As/min-Ab/min) ≦ 0.070 Abs /min
計算
活性 (U/mg) = {(ΔA/min)/6.22} × 3.04/0.04 × 1/x| 6.22 : | NADH の340nm におけるミリモル分子吸光係数 (cm2 /μmole) |
| 3.04 : | 反応総液量 (ml) |
| 0.04 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |
