GLUCOSE–6–PHOSPHATE DEHYDROGENASE
[G6PDHⅡ]
from Bacillus sp.
(D–Glucose–6–phosphate: NADP+ 1–oxidoreductase, EC 1.1.1.49)
D–Glucose–6–phosphate + NADP+ →
D–Glucono–δ–lactone–6–phosphate + NADPH + H+
Preparation and Specification
- Appearance
- : White amorphous powder, lyophilized
- Specific activity
- : More than 100 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 342 kDa (gel filtration)
- Isoelectric point
- : pH 6.13
- Michaelis constants
- : NADP+ 8.3 × 10-6M
G–6–P 1.2 × 10-4M
- Optimum pH
- : pH 8.4 (Tris–HCl) Figure 1
- pH stability
- : pH 6.0–8.0 (75℃, 15 min) Figure 2
- Optimum temperature
- : 75℃Figure3
- Thermal stability
- : Stable at 65℃ and below (pH 7.5, 15 min) Figure4
- Effect of various
chemicals - : See Table 2
- Inhibitors
- : Mn2+, Cu2+, Al3+
- Stabilizer
- : BSA
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of glucose or ATP when coupled with hexokinase (T–50) .
| HK Ⅱ | ||
| D-Glucose + ATP | → | D-Glucose-6-phosphate + ADP |
| G6PDH Ⅱ | ||
| D-Glucose-6-phosphate + NADP+ | → | D-Glucono-δ-lactone-6-phosphate+ NADPH + H+ |
Table 1. Substrate specificity
| Substrate | Relative activity (%) |
|---|---|
| Glucose-6-phosphate | 100 |
| Galactose-6-phosphate | 16 |
| Mannose-6-phosphate | 33 |
| Fructose-6-phosphate | 0 |
| Glucose-1-phosphate | 0 |
Table 2. Effect of various chemicals on G6PDH II activity
| Additives | Consentration | Relative activity (%) |
|---|---|---|
| None | – | 100 |
| NaCI | 10mM | 100 |
| KCI | 10mM | 100 |
| LiCI | 1mM | 100 |
| MgCI2 | 10mM | 100 |
| CaCl2 | 10mM | 100 |
| BaCl2 | 10mM | 97 |
| MnCl2 | 1mM | 42 |
| EDTA | 1mM | 100 |
| CuCl2 | 1mM | 22 |
| Triton X-100 | 1% | 155 |
| Adekatol PC-8 | 1% | 161 |
| Nikkol OP-10 | 1% | 155 |
| Tetronic 704 | 1% | 117 |
Fig.1 Optimum
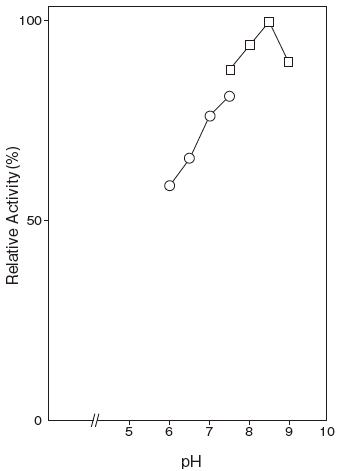
〇: Phosphate buffer
□: Tris-HCI buffer
Fig.2 pH Stability
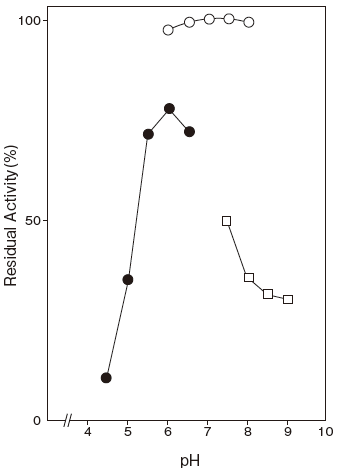
〇: Phosphate buffer
●: 3,3-Dimethylglutarate-NaOH buffer
□: Tris-HCI buffer
Fig.3 Optimum Temperature
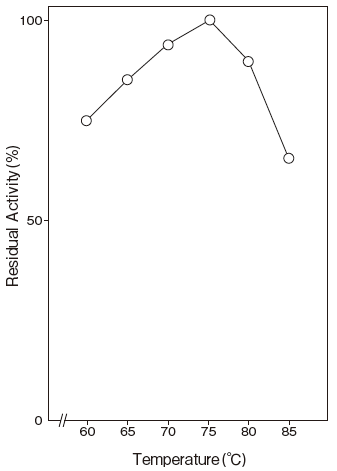
40mM Phosphate buffer
Fig.4 Thermal Stability
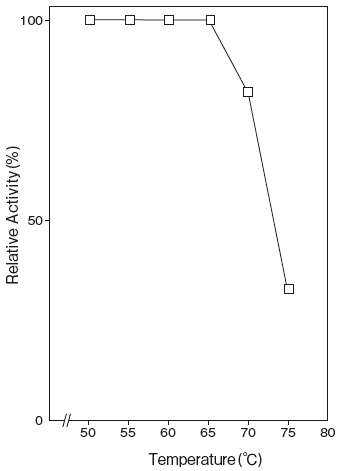
40 mM Tris-HCI buffer
Assay
Principle
The assay is based on the increase in absorbance at 340 nm as the formation of NADPH proceeds in the following reaction:
| G6PDH Ⅱ | ||
| D–Glucose–6–phosphate+NADP+ | → | D–Glucono–δ–lactone–6–phosphate+ NADPH+ H+ |
NADP: Nicotineamide adenine dinucleotide phosphate
Unit definition
One unit is defined as the amount of enzyme which oxidizes 1 μmole of D–glucose–6–phosphate to D–glucono–δ–lactone–6–phosphate per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
0.2 M KH2PO4–K2HPO4 buffer pH 7.5 1.5 ml 2.0% (W/V) BSA solution 0.3 ml 10 mM NADP solution 0.3 ml 0.1 M D–Glucose–6–phosphate solution 0.3 ml Distilled water 0.6 ml - Enzyme dilution buffer
10 mM KH2PO4–K2HPO4 buffer pH 7.5 - Reagents
NADP (oxidized form) :
FUJIFILM Wako Pure Chemical Corporation #308–50463
D–Glucose–6–phosphate: Sigma Chemical Co. #G–7250
BSA: Millipore Fraction V pH5.2 #81–053
Enzyme solution
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration as required.
Procedure
- Pipette accurately 3.0 ml of reaction mixture into a small test tube and preincubate at 37℃.
- After 5 min, add exactly 50 μl of enzyme solution and mix to start the reaction at 37℃.
※ In the case of a test blank, add 50 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase per minute in absorbance at 340 nm. The rate must be measured within the linear portion of the absorbance curve.
△A/min = As/min-Ab/minAbsorbance sample : As/min blank : Ab/min
0.030 Abs/min ≦ △A/min ≦ 0.050 Abs/min
Calculation
- Activity (U/mg of powder) = {(△A/min)/6.22} × 3.05/0.05 × 1/x
6.22 : millimolar extinction coefficient of NADPH at 340 nm (cm2/ μmole)
3.05 : final volume (ml) 0.05 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution (mg/ml)
Storage
Storage at -20℃ in the presence of a desiccant is recommended.
References
- Haberstich, H. V. and Zuber, H. (1971) Arch. Biochem. Biophys., 144, 245–252.
- Muramatsu, N. (1974) Arch. Microbiol., 98, 275–289.
- Ishaque, A., Milhausen, M., Levy, H. R. (1974) Biochem.
Biophys. Res. Commun., 59, 894–901. - Milhausen, M. and Levy, H. R. (1975) Eur. J. Biochem., 50, 453–461.
- Olive, C., Geroch, M. E. and Leuy, H. R. (1971) J. Biol. Chem., 246, 2043–2057.
- Coe, E. C. and Hsu, L. H. (1973) Biochem. Biophys. Res. Commun., 53, 66–69.
- Olive, C. and Levy, H. R. (1967) Biochemistry., 6, 730–736.
- Metzger, R. P., Metzger, S. A. and Parsons, R. L. ( 1972) Arch. Biochem. Biophys., 149, 102–109.
G6PDH Ⅱ活性測定法 (Japanese)
試薬液
- 反応試薬混合液
0.2M KH2PO4–K2HPO4 緩衝液 pH7.5 1.5 ml 2.0% (W/V) BSA 溶液 0.3 ml 10mM NADP 溶液 0.3 ml 0.1M G–6–P 溶液 0.3 ml 精製水 0.6 ml - 酵素溶解希釈用液
10mM KH2PO4–K2HPO4 緩衝液 pH7.5 - 試薬
NADP (ニコチンアミドアデニンジヌクレオチド・リン酸酸化型) :富士フイルム和光純薬製 #308–50463G–6–P (D–Glucose–6–phosphate) :シグマ製 #G–7250BSA: Millipore 製 Fraction V pH5.2 #81–053
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液で溶解して全容20ml とする。
その液を酵素溶解希釈用液で適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液3.0ml を正確に分注し、37℃で予備加温する。
- 5 分経過後、酵素試料液50 μl を正確に加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液
50 μl を加える。 - 反応開始後、340nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。
求められた吸光度変化の試料液はAs/min、盲検液は
Ab/min とする。
0.030 Abs/min ≦ΔA/min = (As/min−Ab/min)
≦ 0.050 Abs/min
計算
活性 (U/mg) = {(△ A/min)/6.22} × 3.05/0.05 × 1/x| 6.22 : | NADPH の340nm におけるミリモル分子吸光係数 ( cm2/ μmole) |
| 3.05 : | 反応総液量 (ml) |
| 0.05 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |
