CHOLESTEROL ESTERASE [CEBPII]
from Microorganism
(Sterol‒ester acylhydrolase, EC 3.1.1.13)
(Sterol esterase)
Cholesterol Ester + H2O → Cholesterol + Fatty Acid
High liquid stability
Preparation and Specification
- Appearance
- : White to off white lyophilized powder
- Specific activity
- : More than 10.0 U/mg solid
Properties
- Substrate specificity
- : See Table 1
- Molecular weight
- : 63 kDa(SDS‒PAGE)
- Isoelectric point
- : 5.1 (estimated from amino acid sequence)
- Optimum pH
- : 5.6Figure 1
- pH stability
- : 4.0‒8.0 (37°C, 60 min)Figure 2
- Optimum temperature
- : 45℃Figure 3
- Thermal stability
- : Stable at 45°C and below(pH6.0, 30 min) Figure 4
- Liquid stability
- : See Figure 5
- Adsorption on glass
- : See Figure 6
- Effect of coexisting ions
- : See Table 2
- Effect of detergents
- : See Table 3
- Light stability
- : See Table 4
Applications for Diagnostic Test
This enzyme is useful for enzymatic determination of total cholesterol, HDL-C,andLDL-C coupled with cholestreol oxidase (T‒84 and T‒101) .
This enzyme is suitable for assembling in liquid reagens.
| CEBPII | ||
| Cholesterol ester + H2O | → | Cholestero + FFA |
| CON Ⅱ | ||
| Cholesterol + O2 | → | △4 -Cholesten-3-one + H2O2 |
| POD | ||
| 2 H2O2 + 4-AA + Phenol | → | Quinoneimine dye + 4 H2O |
FFA: Free fatty acid
Table 1 Substrate specificity
| Substrate (0.95mM) | Relative activity (%) | |
|---|---|---|
| Cholesterol Acetate | C 2:0 | 0.4 |
| Cholesterol Propionate | C 3:0 | 9.3 |
| Cholesterol Butyrate | C 4:0 | 27.8 |
| Cholesterol Palmitate | C16:0 | 19.8 |
| Cholesterol Stearate | C18:0 | 2.0 |
| Cholesterol Oleate | C18:1 | 100.0 |
| Cholesterol Linoleate | C18:2 | 80.6 |
Table 2 Effect of coexisting ions on CEBPⅡ activity
| Coexisting ion (20 mM) | Relative activity (%) |
|---|---|
| None | 100 |
| NaCl | 101 |
| KCl | 100 |
| NH4Cl | 100 |
| CaCl2 | 101 |
| MgCl2 | 101 |
| MnCl2 | 103 |
| ZnCl2 | 105 |
| EDTA | 102 |
| NaN3 | 100 |
| NaF | 98 |
All samples contain 0.1% BSA
Table 3 Effect of detergents on CEBP II activity
| Detergent (0.1%) | Relative activity (%) |
|---|---|
| None | 100 |
| Triton X-100 | 115 |
| Adekatol TN-100 | 117 |
| Adekatol SO-120 | 142 |
| Newcol-707 | 115 |
| Newcol-710 | 113 |
| Emulgen 120 | 141 |
| Emulgen 705 | 135 |
| Deoxycholic acid | 129 |
| Tauroursodeoxycholic acid | 134 |
| Sodium dodecyl sulfate | 127 |
Table 4 Light stability
| Form of CEBP II | Light source | Residual activity (%) |
|---|---|---|
| Powder | LED | 96.5 |
| Fluorescent | 98.7 | |
| Solution | LED | 94.1 |
| (1mg/ml, water) | Fluorescent | 98.4 |
After 24 hours of incubation
Fig. 1 Optimum pH
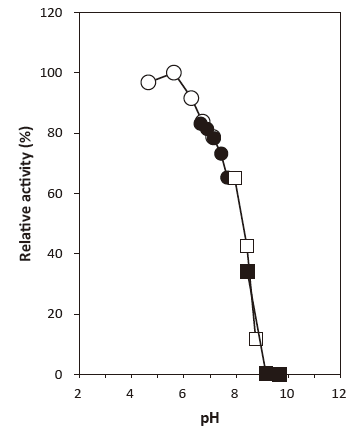
●: Phosphate buffer
□: Tris-HCl buffer
■: Glycine-NaOH buffer
Fig. 2 pH stability
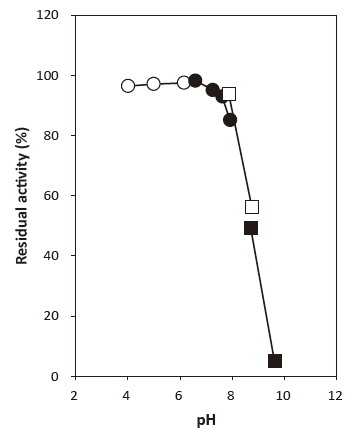
〇: 3.3-Dimethylglutarate-NaOH buffer
●: Phosphate buffer
□: Tris-HCl buffer
■: Glycine-NaOH buffer
Fig. 3 Optimum temperature
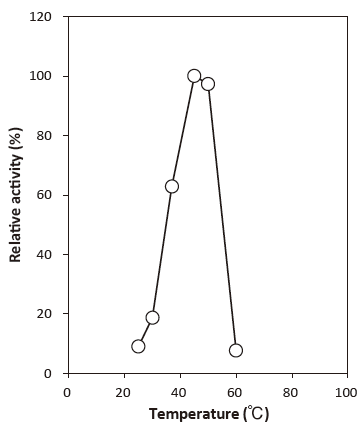
40 mM Phosphate buffer
Fig. 4 Temperature stability
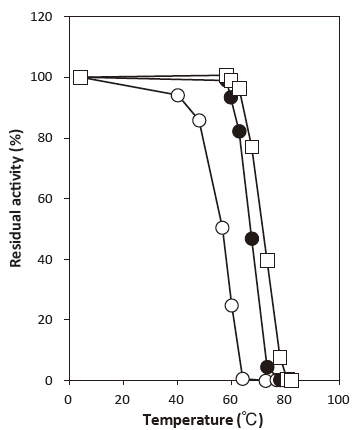
〇: Phosphate buffer
●: Phosphate buffer + 0.1% Emulgen 705
□: Phosphate buffer
+ 0.1% Emulgen 705 & 10 mM NH4Cl
Fig. 5 Stability of CEBPⅡ in a liquid reagent
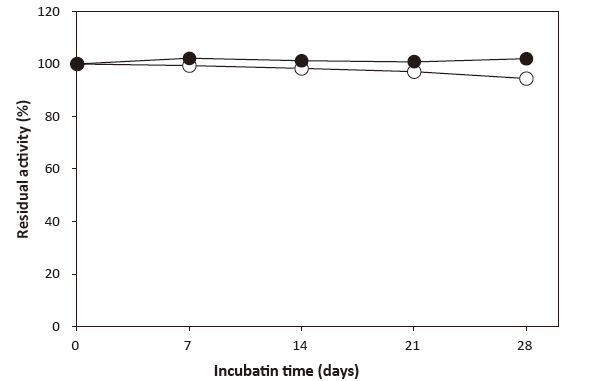
| ● : 4℃ | Reagent composition: | 0.5 U/mL CEBP II |
| ○ : 37℃ | 50 mM BES buffer pH 6.5 | |
| 0.4% Triton X-100 | ||
| 0.05% NaN3 | ||
| 0.6 mM ADOS | ||
| 7.5 U/mL POD |
Fig. 6 Adsorption on glass
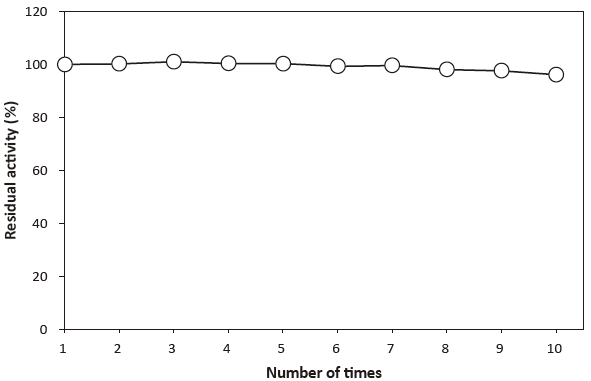
10 mM Phosphate buffer containing 0.1% BSA
Solution of CEBPⅡ was repeatedly transferred into another test tube, and then the residual activity in the solution of each tube was measured.
Assay
Principle
The assay is based on the increase in absorbance at 493 nm as the formation of quinoneimine dye proceeds in the following reactions:
| CEBPⅡ | ||
| Cholesterol ester+H2O | → | Cholesterol+Fatty acid |
| CO | ||
| Cholesterol+O2 | → | △4–Cholesten–3–one+H2O2 |
| POD | ||
| 2 H2O2+4–AA+Phenol | → | Quinoneimine dye+4 H2O |
CO: Cholesterol oxidase
Unit definition
One unit is defined as the amount of enzyme which liberates 1 μmole of cholesterol per minute at 37℃ under the conditions specified in the assay procedure.
Reagents
- Reaction mixture
0.2M KH2PO4‒NaOH buffer pH 6.8 0.60 ml 0.35% 4‒AA solution 0.30 ml 0.2% (W/V) Phenol solution 0.30 ml 100U/ml POD solution1) 0.30 ml 3% (W/V) Triton X‒100 solution 0.30 ml 0.2U/ml CONⅡ solution2) 0.60 ml Substrate solution3) 0.30 ml Distilled water 0.30 ml 1) : 100U/ml POD solution
Dissolve 1000 U (PPU) of POD with 10 ml of distilled water.2) : 0.2U/ml CONⅡ solution
Dissolve 2 U of CONⅡ with CONⅡ dilution buffer※)※) : CONⅡ dilution buffer
0.1M KH2PO4‒Na2HPO4 buffer pH 7.0 containing
0.05% (W/V) Triton X‒100.3) : Substrate solution
Calf serum - Enzyme dilution buffer
10mM KH2PO4‒NaOH buffer pH 7.5 containing 0.1%
(W/V) BSA. - Reagents
Triton X‒100: The Dow Chemical Company
CONⅡ : Nagase Diagnostics Co., Ltd. #T‒84
Calf serum: GIBCO Co. (USA)
BSA: Millipore Fraction V pH5.2 #81‒053
4‒AA: NACALAI TESQUE, INC. Special grade#01907‒52POD: Sigma Chemical Co. Type Ⅱ #P‒8250
Enzyme solution
Accurately weigh about 20 mg of the sample and add enzyme dilution buffer to make a total of 20 ml. Dilute it with enzyme dilution buffer to adjust the concentration to within 0.35 U/ml.
Procedure
- Pipette accurately 3.0 ml of reaction mixture into a small test tube and preincubate it at 37℃.
- After 10 min, add 50 μl of enzyme solution and mix to
start the reaction at 37℃.※ In the case of a test blank, add 50 μl of enzyme dilution buffer in place of enzyme solution. - After starting the reaction, measure the rate of increase per minute in absorbance at 493 nm. The rate must be measured within the linear portion of the absorbance
curve.
△A/min = (As/min-Ab/min) ≦0.040 Abs/minAbsorbance sample : As/min blank : Ab/min
Calculation
- Activity (U/mg of powder) = {(△A/min)/(12.0 × 1/2)} × 3.05/0.05 × 1/x
12.0 : millimolar extinction coefficient of quinoneimine dye
at 493 nm (cm2/ μmole)1/2 : a multiplier derived from the fact that 2 mole of
H2O2 produce 1 mole of quinoneimine dye3.05 : final volume (ml) 0.05 : volume of enzyme solution (ml) X : concentration of the sample in enzyme solution ( mg/ml)
Storage
Storage at -20℃ in the presence of a desiccant is recommended.
References
- Bradford, M. B., (1976) Anal. Biochem., 72, 248‒254.
- Allain, C. C., Poon, L. S., Chan, C. S. G., Richmond, W. and Fu, O.C. (1974) Clin. Chem., 20, 470‒475.
- Kameno, Y., Nakano, N. and Baba, S. (1976) Jap. J. Clin. Path., 24, 650.
CEBPⅡ 活性測定法 (Japanese)
試薬液
- 反応試薬混合液
0.2M2KHPO4‒NaOH 緩衝液 pH6.8 0.60 ml 0.35% 4‒AA 溶液 0.30 ml 0.2% (W/V) フェノール溶液 0.30 ml 100U/ml POD 溶液 1) 0.30 ml 3% (W/V) トリトンX‒100 溶液 0.30 ml 0.2U/ml CONⅡ溶液 2) 0.60 ml 基質溶液 3) 0.30 ml 精製水 0.30 ml 1) : 100U/ml POD 溶液
POD1,000 単位 (PPU) を精製水10ml で溶解する。2) : 0.2U/ml CONⅡ溶液
CONⅡ 2 単位 (U) をCONⅡ溶解用液※) 10ml で溶解する。※) : CONⅡ溶解用液
0.05% (W/V) トリトンX‒100 を含む0.1M
KH2PO4‒Na2HPO4 緩衝液 pH7.03) : 基質溶液
仔牛血清液 - 酵素溶解希釈用液
0.1% (W/V) BSA を含む10mM KH2PO4‒NaOH 緩衝液 pH7.5 - 試薬
トリトンX‒100:Dow Chemical 社製
CONⅡ (コレステロール酸化酵素) :ナガセダイアグノスティックス製 #T‒84仔牛血清液 (Calf serum) : GIBCO (USA) 製
BSA: Millipore 製 Fraction V pH5.2 #81‒053
POD:シグマ製 Type Ⅱ #P‒8250
4‒AA:ナカライテスク製 特級 #01907‒52
酵素試料液
- 検品約20mg を精密に量り、酵素溶解希釈用液に溶解して全容20ml とする。
その液を酵素溶解希釈用液で約0.35U/ml 濃度となるように適宜希釈する。
測定操作法
- 小試験管に反応試薬混合液を3.0ml 正確に分注して37℃で予備加温する。
- 10 分経過後、酵素試料液50 μl を正確に加えて混和し、37℃で反応を開始する。
※ 盲検は酵素試料液の代わりに酵素溶解希釈用液50μl を加える。 - 反応開始後、493nm における吸光度を測定して直線的に反応している1 分間当たりの吸光度変化を求める。
求められた吸光度変化を試料液はAs/min、盲検液はAb/min とする。
Δ A/min = (As/min-Ab/min) ≦ 0.040Abs/min
計算
活性 (U/mg)= {(ΔA/min)/(12.0 × 1/2)} × 3.05/0.05 × 1/x| 12.0 : | キノンイミン色素の493nm におけるミリモル分子吸光係数 (cm2 /μmole) |
| 1/2 : | H2O22 モルからキノンイミン色素1 モルが生成することによる係数 |
| 3.05 : | 反応総液量 (ml) |
| 0.05 : | 反応に供した酵素試料液量 (ml) |
| X : | 酵素試料液中の検品濃度 (mg/ml) |
