
About Us
Who We Are
Accurate diagnosis is important not only for treatment of diseases but also for maintenance of health.
Nagase Diagnostics Co., Ltd. develops, manufactures, and provides in vitro diagnostic products and the enzymes used in these products to ensure dependable and consistent diagnostic performance.
We are committed to delivering healthcare-related products that meet the changing needs of society, from our R&D and manufacturing base in Ōhito, Shizuoka, with a view of Mt. Fuji.
What Nagase Diagnostics offers
Development & manufacturing of more than 100 enzymes supporting the “core of diagnostic products”
We utilize our uniquely developed genetic recombination and microbial cultivation technologies to manufacture and sell enzymes used in various diagnostic products, such as those for blood sugar, lipids, kidney function, and liver function.
We respond to emerging needs in our world as one of the few companies that offer everything from raw enzyme materials to diagnostic products.
about the enzymes

In-vitro diagnostic products that utilize our unique enzyme method
We developed the Lucica™ Glycated Albumin assay kit (liquid reagent), which has been manufactured and sold in Japan since 2004. In 2022, we launched a new Glycated Albumin assay kit as a reagent that meets the need for a reference standard material for measurement of glycated albumin. Lucica™ MI Assay Kit, a myo-Inositol measuring reagent, is a testing agent that uses a urine sample to screen for impaired glucose tolerance, which cannot be detected by fasting plasma glucose (FPG) testing alone.
Lucica™ Glycated Albuminassay kit

Our History
Built upon the brewing techniques instilled within our DNA from
having started as a sake brewery
Nagase Diagnostics Co., Ltd. originally started from the Asahi Kasei Group and Toyo Jozo Co., Ltd., which was the successor to the sake brewery Wakita Shuzo.
The Ohito district of Izunokuni-shi in Shizuoka Prefecture, where our main manufacturing and research sites are located, was originally a sake brewing factory.
The brewing techniques from that time have served as the keystone for developing and manufacturing enzymes and the diagnostic products that use them.
The brewing techniques that have been accumulated for nearly a century are still engraved in the DNA of the people who work there.

- 1920
- Establishment of Toyo Jozo Co., Ltd., successor to sake brewery Wakita Shuzo


main product at the time
- 1947
- Penicillin launchedWe started manufacturing and selling antibiotic agents, which would later become one of our major business lines. We made use of our fermentation techniques to enable us to be among the first to begin researching enzymes and developing diagnostic products.
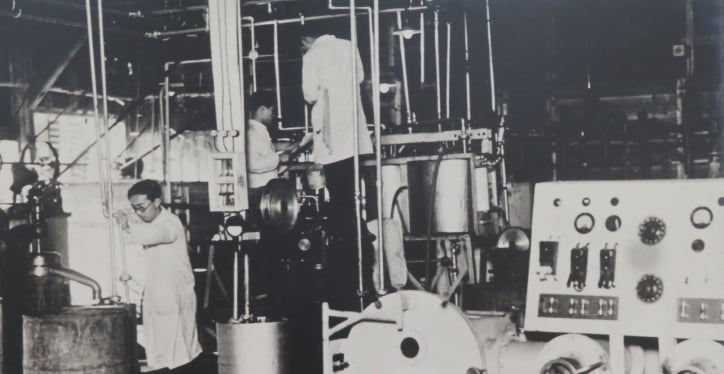
- 1958
- Asahi Kasei acquired capital in Toyo Jozo Co., Ltd.
- 1974
- Development of enzyme for triglyceride measurementThe first enzyme developed by Asahi Kasei Pharma was lipase (T-01), which is used to measure triglycerides.
It was developed as a by-product from the production of penicillin.
The production number “T-01” comes from the first letter in Toyo Jozo.

- 1976
- Development of enzyme cycling methodThe sensitivity limit of regular enzyme assays was said to be 10-⁵mol/L. However, we were the first to aim for and start developing highly sensitive diagnostic drugs that employ an enzyme cycling method that uses cycling reactions to quantify amplified target substance. We have proposed a measurement method using this enzyme cycling method. (Bile acids, ketone bodies, carnitine fraction, myo-Inositol, ammonia, etc.)
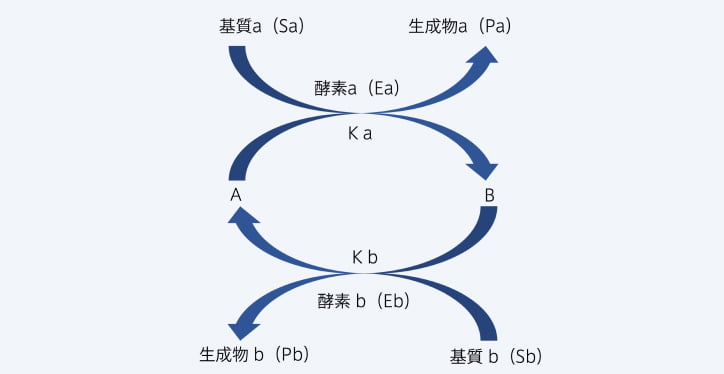
measurements

completed
- 1982
- Ohito headquarters building (current district building) completed
- 1992
- Development & sales of human enzymes through genetic
engineering technologiesWe succeeded in developing and achieving the stable manufacture of eight types of genetically engineered human enzymes that are required to promote standardization to obtain the same results from the same sample, regardless of location, time or person measuring. They have been adopted as reference enzymes by the Japanese Committee for Clinical Laboratory Standards (JCCLS). These products are helping to improve accuracy in measuring enzymes that used to have large variations in values.
- 1992
- Merger of Toyo Jozo Co., Ltd. and Asahi Chemical Industry Co., Ltd.

- 1993
- Development of measurement column for glycated albumin in the bloodWe developed a column for measuring glycated albumin in the blood. This column, along with related measuring equipment, was launched by Kyoto Daiichi Kagaku as a new glycated albumin measurement system.
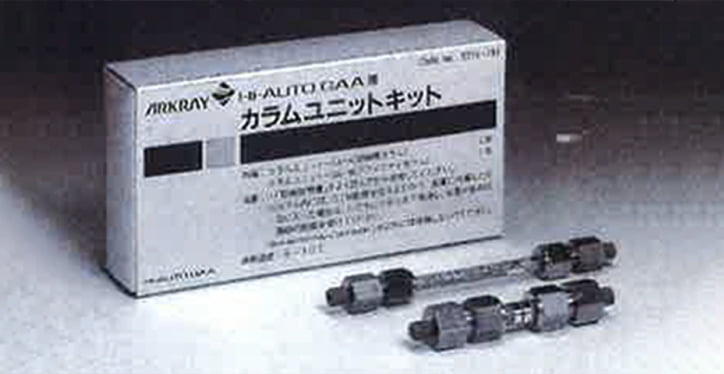
- 2001
- Change of company name to Asahi Kasei Corporation
- 2003
- Established as Asahi Kasei Pharma Corporation following the transition to a "split company/holding company configuration”

- 2004
- Launch of the Lucica™ Glycated Albumin Assay Kit (Liquid Reagent)
in JapanWe launched Lucica™ Glycated Albumin assay kit that utilizes our unique enzyme method. This liquid reagent is used for monitoring blood glucose control over the preceding two to three weeks in diabetes.

measurement in Japan
- 2008
- Launch of Lucica™ MI Assay Kit for Myo-Inositol measurement in JapanLucica™ MI assay kit was launched as Japan’s first in-vitro diagnostic product for measuring myo-Inositol (MI) in urine. This reagent helps screen for impaired glucose tolerance, which cannot be determined by fasting plasma glucose (FPG) testing alone.

- 2022
- Launch of the New Lucica™ Glycated Albumin Assay Kit in JapanIn response to standardization of glycated albumin being promoted by the Committee on Diabetes Mellitus Indices of the Japan Society of Clinical Chemistry, the new Lucica™ Glycated Albumin assay kit was launched, which ensures traceability to reference standard material JCCRM611.

- 2025
- Establishment of Nagase Diagnostics Co., Ltd.As of July 1, 2025, Diagnostics Business, Ohito Pharmaceuticals Plant and Ohito Office of Asahi Kasei Pharma Corporation have been transferred to NAGASE Group and has begun operations as Nagase Diagnostics Co., Ltd.
